Record-high room temperature thermoelectric figure of merit for metal oxides
Yugo Takashima, Yu-qiao Zhang*, Jiake Wei, Bin Feng, Yuichi Ikuhara, Hai Jun Cho, and Hiromichi Ohta*, “Layered cobalt oxide epitaxial films exhibiting thermoelectric ZT = 0.11 at room temperature”, J. Mater. Chem. A (2020). (October 13, 2020) (DOI: 10.1039/D0TA07565E)
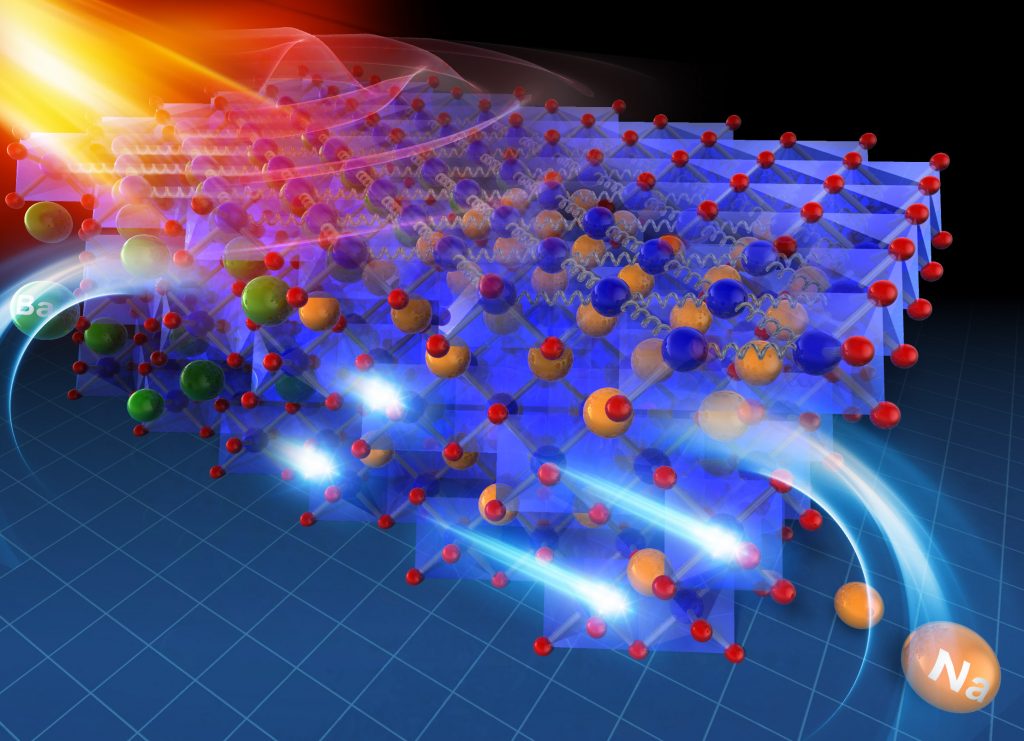
A research group of Professor Hiromichi Ohta at the Research Institute for Electronic Science, Hokkaido University has realized layered cobalt oxide showing a record high thermoelectric figure of merit ZT = 0.11 at room temperature. Thermoelectric conversion is attracting attention as a technology for reusing waste heat discharged from factories and automobiles. Metallic chalcogenides such as PbTe are known as thermoelectric materials, but they have not been put into practical use on a large scale due to their problems of thermal and chemical stability and toxicity. Compared to metal chalcogenides, many metal oxides are stable even at high temperatures in air. Therefore, they have been studied as thermoelectric materials in Japan for about 25 years. The conversion efficiency of thermoelectric materials is represented as the figure of merit ZT [=(thermopower)2×(electrical conductivity)×(absolute temperature)÷(thermal conductivity)]. The ZT of p-type PbTe is about 0.1 at room temperature. Among the metal oxides, the layered cobalt oxide containing sodium ions between layers exhibits high thermopower and high electrical conductivity. Although Na0.75CoO2 is expected as a practical thermoelectric material, the ZT was only about 0.03 due to its high thermal conductivity. Prof. Ohta’s research group replaced the sodium ions of the layered cobalt oxide with various metal ions, and found that the thermal conductivity decreased as the metal ion layer became heavier without reducing the electrical properties. Finally, they found that ZT reached 0.11 at room temperature when replaced with heavy barium ions. In general, the figure of merit ZT increases as the temperature rises. Therefore, it is expected that a stable and practical thermoelectric conversion material will be realized. The results have been published online as “Advanced Manuscript” in the Journal of Materials Chemistry A on October 31, 2020.
【Information of the paper】
Title: Layered cobalt oxide epitaxial films exhibiting thermoelectric ZT = 0.11 at room temperature
Authors: Yugo Takashima, Yu-qiao Zhang*, Jiake Wei, Bin Feng, Yuichi Ikuhara, Hai Jun Cho, and Hiromichi Ohta*
Journal: Journal of Materials Chemistry A(IF = 11.301)
DOI: 10.1039/D0TA07565E
Published: October 13th, 2020 (Accepted Manuscript)
【Background】
Thermoelectric conversion is attracting attention as a technology for reusing waste heat discharged from factories and automobiles. Metallic chalcogenides such as PbTe are known as thermoelectric materials, but they have not been put into practical use on a large scale due to their problems of thermal and chemical stability and toxicity. Compared to metal chalcogenides, many metal oxides are stable even at high temperatures in air. Therefore, they have been studied as thermoelectric materials in Japan for about 25 years. The conversion efficiency of thermoelectric materials is represented as the figure of merit ZT [=(thermopower)2×(electrical conductivity)×(absolute temperature)÷(thermal conductivity)]. The ZT of p-type PbTe is about 0.1 at room temperature. Among the metal oxides, the layered cobalt oxide containing sodium ions between layers exhibits high thermopower and high electrical conductivity. Although Na0.75CoO2 is expected as a practical thermoelectric material, the ZT was only about 0.03 due to its high thermal conductivity.
【Approach】
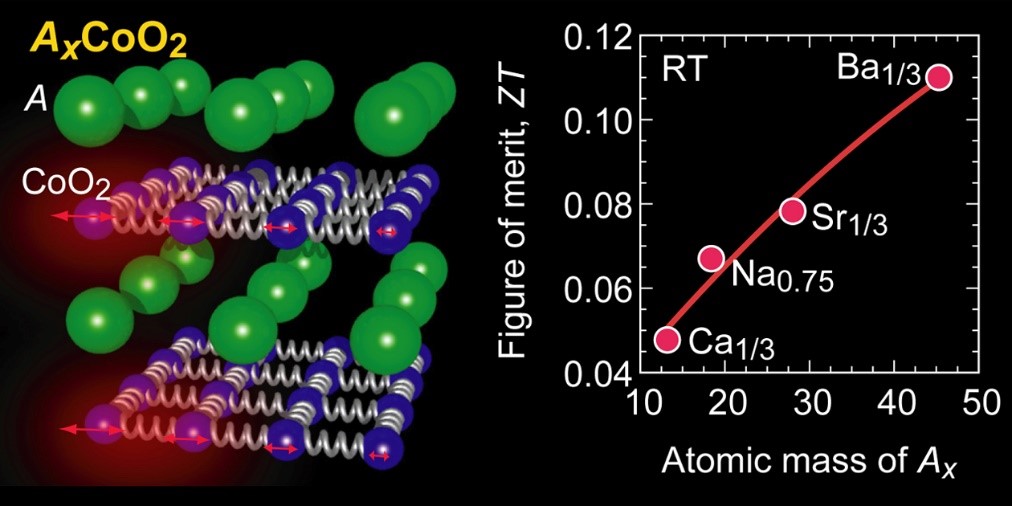
The research group considered the strategy as shown in Fig. 1 to reduce the thermal conductivity of the layered cobalt oxide AxCoO2. The crystal structure of AxCoO2 is a layered structure in which A ion layers and CoO2 layers are alternately laminated. It is known that the thermoelectric conversion performance is higher along the layer than in the direction perpendicular to the layer. Heat conduction in the direction along the AxCoO2 layer is mainly caused by the propagation of atomic vibrations in the CoO2 layer. When the A ion is light, the vibration of the CoO2 layer propagates without attenuation, so the thermal conductivity is high, but we hypothesized that the heavier A ion would immediately attenuate the vibration of the CoO2 layer.
Here, we briefly explain heat conduction. Assuming that the vibration of each atom is a “spring”, heat propagation is similar to the propagation of expansion and contraction of a “spring”. Let’s say, you have a mattress with lots of “springs”. When a baby is placed on the mattress, the “spring” of the mattress is not affected, but when a large sumo wrestler rides, the “spring” contracts and becomes immobile. From these considerations, we predicted that the thermal conductivity could be reduced by making the A ion heavier.
The research group firstly prepared a Na0.75CoO2 thin film, and then prepared an AxCoO2 thin film in which Na0.75 was replaced with Ca1/3, Sr1/3, and Ba1/3, which have different weights, by the ion exchange method. As a result of structural analysis of the prepared thin film, it was found that the Na ions of the Na0.75CoO2 thin film were replaced with Ca1/3, Sr1/3, and Ba1/3. After that, the electrical conductivity, thermopower, and thermal conductivity were measured at room temperature.
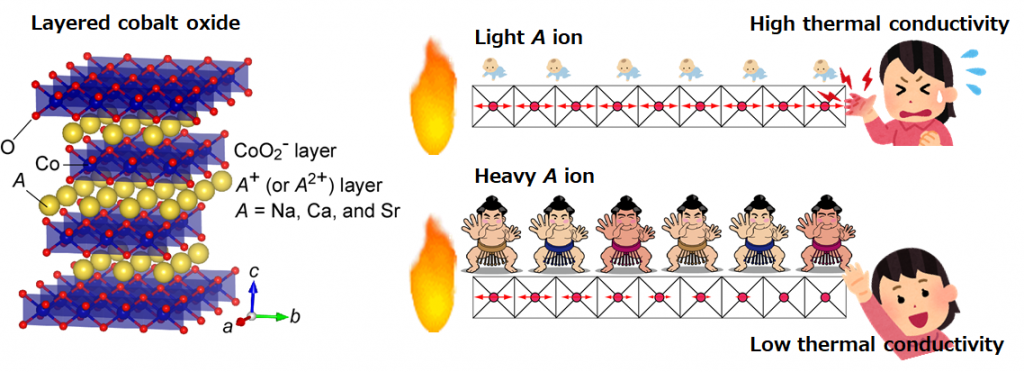
Figure 1. Strategy for reducing the thermal conductivity of layered cobalt oxide AxCoO2.
【Results】
Figure 2 summarizes the thermoelectric properties in the direction parallel to the layer of the Ax-substituted AxCoO2 thin film measured at room temperature. The electrical conductivity and thermopower is slightly affected by the substitution of A ions, but the power factor represented by [(thermopower)2×(electrical conductivity)] is almost constant regardless of Ax. Since the current flows through the CoO2 layer, it can be said that the substitution of Ax ions does not affect the electrical properties. On the other hand, the thermal conductivity decreases monotonically as the Ax atomic weight increases. Ba is the heaviest element you can choose between alkali metals and alkaline earth metals. It was found that only this decrease in thermal conductivity was directly reflected in the change in the thermoelectric figure of merit, and that Ba1/3CoO2 substituted with Ba reached the maximum 0.11 thermoelectric conversion performance index for oxides at room temperature.
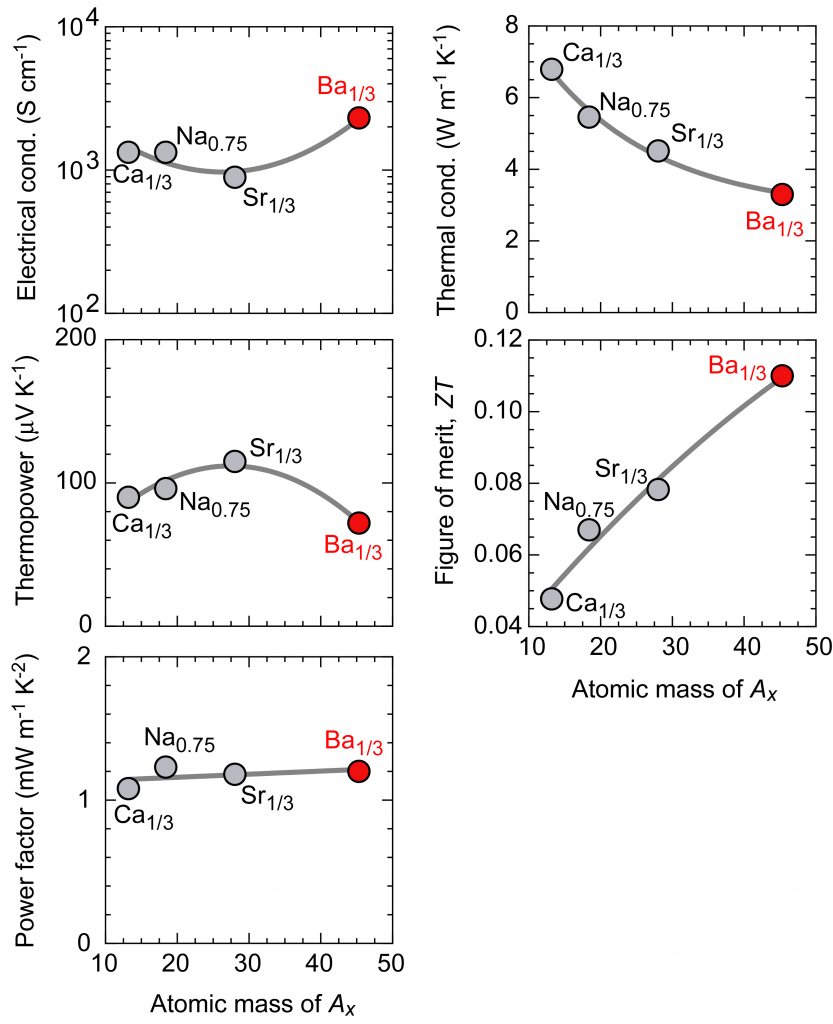
Figure 2. Thermoelectric properties (room temperature) in the direction parallel to the AxCoO2 thin film layer in which Ax is replaced.
【Future expectation】
In general, the figure of merit ZT increases as the temperature rises. Currently, we are also measuring the thermoelectric characteristics at high temperatures, and it has been confirmed that ZT rises. In the future, it is expected that stable and practical thermoelectric conversion materials will be realized by further optimizing the composition and enhancing the thermoelectric conversion performance.
【Acknowledgements】
This research was supported by Grants-in-Aid for Innovative Areas (19H05791 and 19H05788) and Grants-in-Aid for Scientific Research A (17H01314) from the JSPS. A part of this work was supported by Dynamic Alliance for Open Innovation Bridging Human, Environment, and Materials, and by the Network Joint Research Center for Materials and Devices. A part of this work was conducted at Advanced Characterization Nanotechnology Platform of the University of Tokyo, supported by “Nanotechnology Platform” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, Grant Number JPMXP09A20UT0090. H.J.C. acknowledges the support from Nippon Sheet Glass Foundation for Materials Science and Engineering. Y.Z. acknowledges the support from the International Research Fellowship from the JSPS (19F19049).
【Contact】
Prof. OHTA, Hiromichi (Research Institute for Electronic Science, Hokkaido University)
TEL +81-11-706-9428
FAX +81-11-706-9428
メール hiromichi.ohta(at)es.hokudai.ac.jp
URL https://functfilm.es.hokudai.ac.jp/english/

